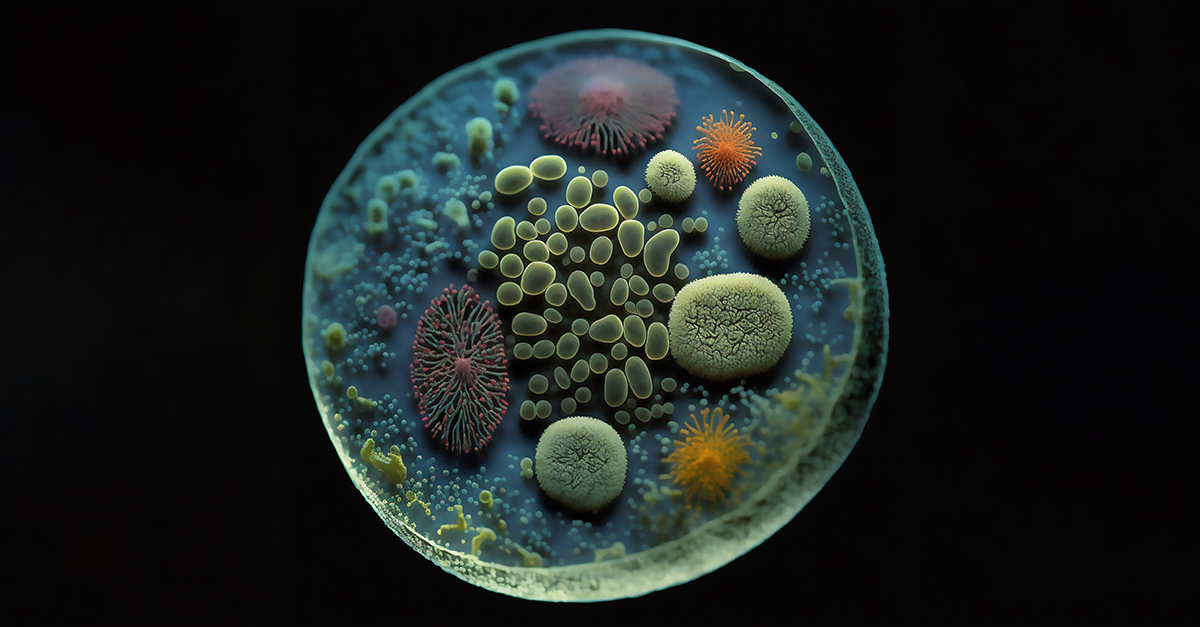
Even if you are not familiar with the term biofilm, you have certainly encountered biofilms on a regular basis. For example, the plaque that forms on your teeth and causes tooth decay is one type of bacterial biofilm. The “gunk” that clogs your household drains is also a biofilm. A persistent infection on a scrape from a sports injury is likely a biofilm.
Biofilms present a significant challenge in maintaining clean and healthy environments in the built environment. Estimates link between 65% and 80% of all bacterial and chronic infections to biofilms, according to research published in the Journal of Chinese Medicine in 2018.
Beyond health concerns, the presence of biofilm on a surface can cause material degradation, fouling that impedes fluid flow or heat transfer, and cosmetic discoloration. Increasingly recognized as a critical global issue in a multitude of industries, biofilms result in estimated economic costs of US$5 trillion annually, according to research published in the journal npj Biofilms and Microbiomes in 2022.
What is a biofilm?
Biofilms are clusters of living, reproducing microorganisms that adhere to just about any type of surface—wet or dry. Biofilms often contain various types of microorganisms such as bacteria,
algae, fungi, yeasts, and protozoa.
Biofilm colonies can form on floors, walls, fixtures, furniture, equipment, medical implants, kitchen counters, contact lenses, and the walls of a hot tub or swimming pool. They adhere to metal,
plastics, and natural materials—such as rocks—as well as human and animal tissue.
These microorganism colonies secrete extracellular polymeric substance (EPS), a slimy, glue-like matter composed of polysaccharides, proteins, nucleic acids, and lipids that provides structural support around the biofilm. EPS works as a protective barrier to make the biofilm’s bacteria and fungi resistant to cleaning products, disinfectants, and antimicrobials.
As biofilms mature, they disperse cells to colonize other surfaces. Biofilms can grow to become many inches thick, such as the biofilms made up of bacteria, fungi, and yeasts often found in pipes or HVAC systems. Biofilms can also be just a few cell layers thick, thin enough to avoid detection by the naked eye. For example, biofilms that can grow on kitchen counters or cell phones
are generally undetectable to the naked eye.
Fortunately, cleaning professionals can use hand-held ultraviolet (UV) lamps to detect hard-to-see biofilms. These lamps use specific wavelengths that pick up components of the microorganisms within the biofilm through a distinctive fluorescent glow.
How can you remove biofilms from surfaces?
Bacteria within biofilms are more difficult to remove using a wet cloth compared to bacteria outside of biofilms. In a study published in the Journal of Hospital Infections in 2019, researchers
found they could wipe away 99.99% of a bacteria culture from a stainless-steel surface with a single swipe of a wet cloth. After five wipes, they had removed 99.999% of the bacteria culture.
Researchers then grew the same bacteria in a dry-surface biofilm. After 50 wipes with a wet cloth, they removed a little over 96% of the bacteria culture from the surface. Researchers determined that biofilm bacteria are 100 times harder to remove than bacteria that is not contained in a biofilm.
A study on cleaning hospital surfaces published in the journal Hygiene in 2022 noted several challenges in removing biofilms:
- Eradicating all surface microbes, whether part of a biofilm community or not, will encourage the rapid repopulation of a newly decontaminated surface with environmental, and other, microbes.
- Disrupting wet surface biofilms by exposing them to bleach resulted in increased detection of multidrug resistant-organisms including carbapenemase-producing Enterobacteriaceae.
- Disinfectant use is more likely to encourage multidrug resistant pathogens such as vancomycin-resistant enterococci, Acinetobacter spp., and Gram-negative organisms such as Stenotrophomonas spp. due to the selection of linked biocidal and antimicrobial resistance characteristics.
- Hospitals that rely upon detergent or probiotic-type products encourage the repopulation of surfaces with inert environmental organisms such as Methylobacteria and Bacillus spp.
Why is it important to keep up with biofilm research?
Researchers have studied the effective treatment (i.e., destruction) of harmful microorganisms, such as bacteria, for many years, however, they looked at it from the wrong perspective. They
studied harmful microorganisms in isolation as individual organisms, not as members of a biofilm colony, where they normally reside.
Cleaning products—such as a disinfectants—are advertised to “kill millions of germs on contact.” This kill claim is considered a truthful statement proven by research. But how accurate was the research? Consider those millions of germs embedded in a biofilm and protected by EPS. More recent studies have shown that germs within a biofilm demonstrate a remarkable resistance to cleaning and disinfecting.
In the development of cleaning and disinfecting products, researchers test their effect on harmful microorganisms in a planktonic state—a state in which microorganisms float in a solution as individuals, not as part of a biofilm colony attached to a surface. So, although such treatments may indeed kill millions of germs on contact, the effect of these treatments is severely limited when they are applied to real world environments in which the microorganisms are members of a biofilm.
For example, a 2023 study published in the journal Antimicrobial Resistance & Infection Control found that:
- The bacterium bacillus subtilis, a strong EPS producer, is resistant to several active ingredients in disinfectants including chlorine dioxide (0.03% concentration), hydrogen peroxide (7.5%), and peracetic acid (2.25%). When in a biofilm, the bacterium also protects the pathogen Staphylococcus aureus from peracetic acid (0.35%).
- The bacterium Acinetobacter johnsonii, when in a biofilm, protects the pathogen Salmonella enterica subsp. enterica serovar Liverpool against benzalkonium chloride (300 mg/L).
Who wants to be a biofilm buster?
The study of biofilms represents a radical new way of understanding the microbiology of virtually everything around us, including issues that affect the cleaning industry and cause serious public health problems. Along with this new understanding comes an exciting opportunity for new business—to rethink our strategies for dealing with biofilms and create new solutions for eliminating the harmful microorganisms within them.
For more information, watch a video on biofilms.